10 years ago, we set out as pioneers with a bold vision: to empower individuals and healthcare providers with an innovative tool that could detect heart rhythm disorders using nothing more than a smartphone. Today, as we celebrate ten years of FibriCheck, we reflect on how our teamwork has transformed the landscape of cardiac health, providing millions with the means to monitor their heart health anytime, anywhere. As we look back on the past ten years, we also look forward to what the future holds for FibriCheck and its role in shaping the future of digital health.
2014: The founding of FibriCheck
– Qompium, the company that created FibriCheck, was founded as the result of a Master’s thesis at Hasselt University.
– Qompium was chosen by Bayer Healthcare for the Grants4Apps accelerator programme.
2015: Recognition and clinical research
– Agoria, the Belgian technology industries trade association recognised FibriCheck with an eHealth award in the Mobile Health category.
– Feasibility study launched with the support of the Flemish government.
2016: Quality certification
– FibriCheck received a CE class IIa certification.
– ISO 13485 certification was obtained (quality management system for the design,
development and distribution of medical equipment).
– FibriCheck won the audience award at the Aging 2.0 conference in San Francisco.
– An innovation project was launched with the support of the Flemish government to deploy the world’s first model on a wearable device.
2017: FibriCheck: the world’s first app on prescription
– FibriCheck booked the first paying user and was officially launched as the first app on prescription.
– First major capital round with LRM, Volta Ventures and other private investors.
– FibriCheck was awarded the Best Mobile Health app at the conhIT (Connecting Healthcare IT) conference in Berlin.
– FibriCheck was chosen as an mHealth project as part of FAGG (Federal Agency for Medicines and Health Products of Belgium) and RIZIV (Belgian National Institute for Health and Disability Insurance).
– We were awarded best digital health company at the European Society of Cardiology conference in Barcelona, the world’s largest and most important cardiology conference.
2018: FibriCheck launched for smartwatch
– We received a prestigious venture award from the European Institute of Innovation and Technology (EIT) as the scale-up of the year.
– FibriCheck received the Vodafone Award.
– FibriCheck was awarded ‘most promising KMO of Limburg’.
– We expanded our business model with corporate health offering.
2019: Further expansion of FibriCheck’s business model
– Our first mass detection campaign in the world took place with 65,000 participants recruited through a newspaper. Results of this campaign were presented at both HRS and ESC conferences.
– After receiving feedback from our users, we expanded our offering by launching the FibriCheck B2C model.
– ORCHA, an authority advisor for health applications, rated FibriCheck their highest ranking application, thereby recognizing our leadership.
– FibriCheck expanded to wearable devices and was first in the world to launch clinical grade analytics on wearables together with Fitbit.
– We launched FibriCheck on Samsung Galaxy watches.
2020: A year of innovation and collaborations
– The world recognized the power of digital health, which drove the adoption of our medical technology. The Europe-wide remote monitoring project TeleCheck-AF was launched, enabling over 40 clinical centres in 14 countries to deliver high-quality care during the COVID-19 pandemic.
– We were selected to join the NHS Innovation Accelerator (NIA) and the DigitalHealth.London accelerator programme (DHL).
2021: Expanding our market access internationally and diving deeper into existing markets to offer end-to-end solutions
– Launch of FibriCheck in Australia following approval by the Therapeutic Goods Administration (TGA).
– Approval and launch of FibriCheck by the Health Sciences Authority (HSA) in Singapore.
– The Belgian Heart Rhythm Association (BeHRA) ran a national awareness campaign using FibriCheck, empowering thousands of Belgians to check their own heart health.
– We were mentioned in the new UK hypertension pathway framework guidance and AF pathway framework guidance.
– FibriCheck was recognised in the Primary Care Cardiovascular Society guidance for long-term CVD (cardiovascular disease) management during COVID-19.
– We started our collaboration with Digital Health London, after being accepted into their accelerator programme late 2020.
– We started with end-to-end pathways in the UK, guiding unknown patients to diagnosed results, thereby driving clinical actionability.
2022: First steps towards reimbursement
– Setup of a nationwide managed detection service in the UK.
– Dutch health insurance reimburses FibriCheck in the secondary post-AF management care pathway.
– Belgian health insurers recognised the incredible value that FibriCheck could bring to their members and decided to reimburse our solution.
– Large scale systematic change by reinventing clinical pathways. We provided FibriCheck for palpitation management in more than 150 GP practices in the UK!
– FibriCheck was approved by the Saudi Food and Drug Authority.
– First SDK integration of FibriCheck with the telemonitoring platform Altibbi, expanding our service offering to third party applications.
– Driving physician education through the FibriCheck Academy with currently over 80+ peer reviewed publications.
2023: Significant growth and new product features
-Significant growth as the number of FibriCheck users surpasses 1 million and we forged more than 110 partnerships in various countries all over the world.
-FibriCheck was offered for free to members of certain health insurers.
–Ground breaking improvements in our diagnostic accuracy after training our algorithm on data from hundreds of thousands of users and utilising over millions of measurements.
-We expanded in cardiovascular pathways by providing the ability to manage comorbidities. We started adding new features such as blood pressure logging, weight logging, and treatments and diagnostics logging. This offers healthcare providers more insights into the heart health of their patients, for more informed clinical decision making.
-We did a complete redesign of the FibriCheck Portal after listening to our healthcare providers, thereby streamlining efficiency and actionability.
-We joined forces with the EHRA-PATHS consortium to contribute to comorbidity management. The aim of this consortium was to transform AF clinical practice to a holistic, inclusive and personalised treatment in which we want to see an evolution from treating AF as an arrhythmia-only problem to treating AF as a manifestation and expression of underlying diseases.

2024: Expansion to the U.S.
-We received FDA-clearance for the entire FibriCheck ecosystem, including our user-facing app, our AI-powered algorithm and our portal for healthcare providers.
-We opened a new office in New York and are transitioning to the US, forging new alliances and partnerships by tapping into the network of prestigious accelerator programs, such as the world renowned MedTech Innovator.
-The Essex Cardiothoracic Centre won a Global Cardiovascular Award for our nurse-led, digitally-enabled AF monitoring pathway.
–FibriCheck was integrated in Sanitas, part of the Bupa Group.
-Over 20+ clinical pathways deploying FibriCheck’s technology are now available to make heart healthcare more efficient.
–More than 2500 physicians are prescribing FibriCheck to their patients.
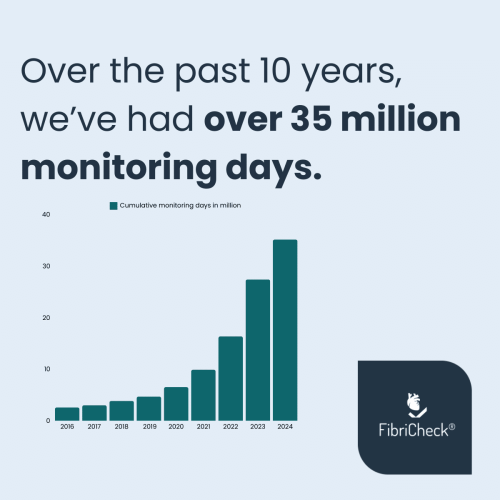
After already having provided 35 million monitoring days to our 1 million+ users, we are looking forward to the future, as we continue our journey to save lives and reduce the burden of cardiovascular diseases. We aim to do so by transforming the way cardiac care is delivered, and by empowering people, patients & caregivers with the right tools and actionable data.
Want to collaborate with us to improve the future of cardiac care?
Created on August 28th, 2024 at 12:48 pm



